Thrombocyte what is a thrombocyte? These tiny blood cells, also known as platelets, play a vital role in our bodies, specifically in the intricate process of blood clotting and hemostasis. They’re crucial for preventing excessive bleeding and are involved in wound healing. Their tiny size belies their significant impact on our overall health. This exploration will unravel the mysteries of these microscopic warriors, from their formation to their functions and the disorders that can affect them.
Thrombocytes, or platelets, are small, irregular-shaped cell fragments, crucial for stopping bleeding. They are produced from megakaryocytes in the bone marrow and circulate in the blood, readily responding to damaged blood vessels. They are key players in primary hemostasis, forming temporary plugs at the site of injury to halt blood loss. Understanding their structure, function, and potential disorders is vital for comprehending their importance in maintaining our body’s health.
Introduction to Thrombocytes
Thrombocytes, also known as platelets, are crucial components of the blood responsible for blood clotting. Their primary function is to initiate the complex cascade of events that lead to the formation of a blood clot, preventing excessive bleeding. This vital process is essential for maintaining vascular integrity and overall health.Platelets, despite their small size, play a significant role in hemostasis, the body’s natural mechanism to stop bleeding.
Understanding their structure and function is key to comprehending how the body maintains a stable internal environment.
Definition and Alternative Name
Thrombocytes are small, irregular-shaped, non-nucleated cell fragments that circulate in the blood. They are derived from megakaryocytes, large cells in the bone marrow. The alternative name, platelets, is more widely used in clinical settings, though thrombocyte is still acceptable and accurate. The term platelet was adopted in the late 19th century when their role in blood clotting was becoming more apparent.
The term reflects the small, disc-like shape of the cellular fragments.
Role in Hemostasis
Thrombocytes are critical for the process of hemostasis, a multi-step process to stop bleeding. They adhere to damaged blood vessel walls and aggregate to form a temporary plug, preventing blood loss. This is just the initial stage; the process then involves complex interactions with other blood components, leading to the formation of a stable blood clot. This process is vital for wound healing and prevents the body from excessive blood loss.
Morphology and Size Range
Thrombocytes are small, irregular-shaped fragments of cytoplasm, typically 2-4 µm in diameter. Their morphology is not uniform; they can vary in shape and size, but are typically biconvex discs. They lack a nucleus and other organelles. The range of size is important in recognizing abnormal platelet counts and associated conditions.
Thrombocytes, also known as platelets, are crucial for blood clotting. Understanding their function helps us appreciate the intricate balance within our bodies. Their role in wound healing, for example, is closely tied to the higher-level cognitive functions of the brain, particularly in the the brains frontal lobe. This area is vital for decision-making, planning, and ultimately, our overall health.
Ultimately, a deep understanding of thrombocytes is essential to appreciating the complex interactions within our physiology.
Comparison to Other Blood Components
| Component | Description | Function | Nucleated |
|---|---|---|---|
| Erythrocytes (Red Blood Cells) | Biconcave discs, filled with hemoglobin | Oxygen transport | No |
| Leukocytes (White Blood Cells) | Various shapes and sizes, with nuclei | Immune response | Yes |
| Thrombocytes (Platelets) | Small, irregular fragments of cytoplasm | Hemostasis (blood clotting) | No |
This table highlights the key differences in structure and function between thrombocytes and other blood components. Understanding these distinctions is crucial for interpreting blood tests and diagnosing various medical conditions.
Thrombocyte Formation and Development
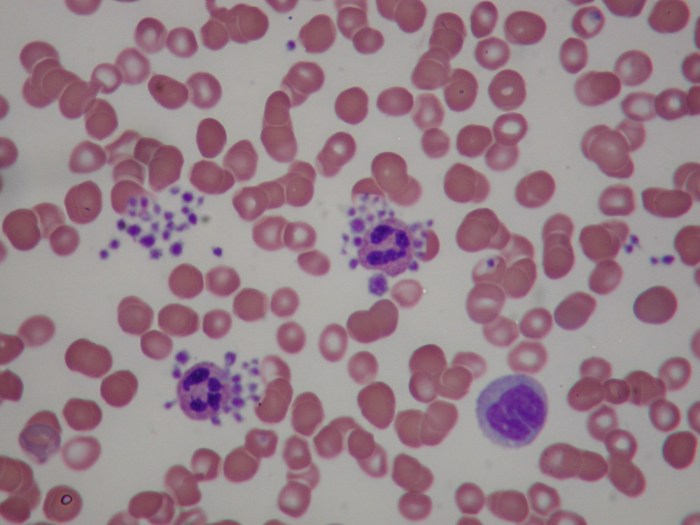
Platelets, also known as thrombocytes, are crucial components of the blood, playing a vital role in hemostasis, the process of stopping bleeding. Their formation, a complex process called thrombopoiesis, is tightly regulated to ensure an adequate supply for blood clotting. This intricate process involves a specific lineage of cells and a precise sequence of events.Thrombopoiesis is a carefully orchestrated process, beginning in the bone marrow, where megakaryocytes, the precursor cells for platelets, differentiate and mature.
The development of platelets from megakaryocytes is a fascinating example of cellular differentiation, exhibiting distinct stages and regulated by specific factors.
Megakaryocyte Lineage
Megakaryocytes are large, polyploid cells, containing multiple copies of the genome. They originate from hematopoietic stem cells in the bone marrow. These cells undergo endomitosis, a unique form of cell division where DNA replicates but cell division doesn’t occur, resulting in an increase in the amount of genetic material within the cell. This process is essential for producing the large quantities of proteins and other molecules needed for platelet formation.
Stages of Thrombocyte Development
Platelet production begins with the development of megakaryocytes, which then fragment into thousands of platelets. This process involves several distinct stages:
- Megakaryocyte Progenitor Cells: These are the early precursors of megakaryocytes, derived from hematopoietic stem cells. They undergo specific genetic changes to adopt the megakaryocytic lineage.
- Megakaryocyte Maturation: Megakaryocytes grow in size and develop characteristic features like a large, lobulated nucleus and abundant cytoplasm. Their cytoplasm becomes filled with organelles and proteins crucial for platelet formation. The maturation process also involves the formation of demarcation membranes that eventually split the cytoplasm into distinct platelet precursors.
- Platelet Budding and Release: As megakaryocytes mature, they extend cytoplasmic protrusions called proplatelets into the bone marrow sinusoids. These proplatelets fragment, releasing thousands of platelets into the bloodstream. The demarcation membranes are essential for this process, as they delineate the boundaries of the developing platelets.
Factors Regulating Thrombopoiesis
Several factors influence the rate of thrombopoiesis, ensuring a balance between platelet production and destruction. These include:
- Thrombopoietin (TPO): This glycoprotein hormone is the primary regulator of thrombopoiesis. TPO levels are crucial for controlling the production of megakaryocytes and platelets. Decreased TPO levels lead to a decrease in platelet production, while increased levels lead to an increase in platelet production.
- Interleukins (ILs) and other Cytokines: These signaling molecules play supporting roles in thrombopoiesis. They can either stimulate or inhibit the production of megakaryocytes, depending on the specific cytokine and the stage of development.
Comparison with Other Blood Cell Types
The maturation of thrombocytes differs significantly from other blood cell types. While other cells, such as erythrocytes and granulocytes, mature within the bone marrow and enter the bloodstream as complete cells, thrombocytes originate from the fragmentation of megakaryocytes. This unique process reflects the specialized function of platelets in hemostasis.
- Erythrocytes: Mature in the bone marrow, then enter the circulation as complete cells, primarily focusing on oxygen transport.
- Granulocytes: Mature in the bone marrow, and enter the circulation as complete cells, with roles in immune response.
Flowchart of Thrombocyte Development
(A simplified visual representation of the process is shown below)“`Hematopoietic Stem Cell –> Megakaryocyte Progenitor Cell –> Megakaryocyte Maturation –> Platelet Budding and Release –> Circulation“`
Structure and Function of Thrombocytes: Thrombocyte What Is A Thrombocyte
Thrombocytes, also known as platelets, are crucial components of the blood’s clotting mechanism. These small, anucleated cell fragments play a vital role in maintaining vascular integrity and preventing excessive blood loss following injury. Understanding their structure and function is essential for comprehending the intricacies of hemostasis.The internal structure of a thrombocyte is remarkably simple yet sophisticated, reflecting its specialized function in blood clotting.
Unlike other blood cells, platelets lack a nucleus, but they are packed with various organelles and components that enable their critical role in primary hemostasis.
Internal Structure and Components
Platelets possess a highly organized internal structure, essential for their rapid response to vascular injury. They contain numerous granules, which are membrane-bound sacs filled with a variety of bioactive molecules. These granules are categorized into alpha granules, dense granules, and lysosomes, each containing distinct sets of proteins crucial for platelet function. The cytoplasm of the platelet is rich in microtubules and microfilaments, providing structural support and enabling shape changes necessary for adhesion and aggregation.
Alpha Granules
These granules store various proteins, including clotting factors, growth factors, and adhesive molecules. These factors are released upon activation, contributing to the coagulation cascade and promoting tissue repair. Examples of proteins found within alpha granules include fibrinogen, von Willebrand factor, and various growth factors.
Dense Granules, Thrombocyte what is a thrombocyte
Dense granules contain molecules vital for platelet activation and aggregation. These include ADP, ATP, serotonin, and calcium ions. The release of these substances amplifies the platelet response to injury and promotes the recruitment of additional platelets to the site.
Cytoplasmic Components
Microtubules and microfilaments form a cytoskeleton within the platelet, maintaining its shape and enabling changes in morphology. These structural components are crucial for the platelet’s ability to adhere to damaged vessel walls and aggregate with other platelets to form a plug.
Mechanisms of Adhesion to Damaged Vessels
Platelet adhesion to damaged blood vessels is a multi-step process initiated by the exposure of subendothelial collagen. von Willebrand factor (vWF), a large multimeric glycoprotein, plays a critical role in this process. vWF binds to exposed collagen and simultaneously to specific receptors on the platelet surface, mediating platelet adhesion.
Role in Primary Hemostasis
Primary hemostasis involves the formation of a platelet plug to temporarily seal the injured blood vessel. Platelets, upon activation, undergo a series of shape changes, becoming more spread and sticky. These activated platelets aggregate, forming a temporary plug that halts bleeding until a more permanent clot is formed.
Platelet Receptors and Their Functions
| Protein/Receptor | Function |
|---|---|
| Glycoprotein Ib (GPIb) | Binds von Willebrand factor (vWF) |
| Glycoprotein IIb/IIIa (GPIIb/IIIa) | Mediates platelet aggregation |
| Integrins | Mediate adhesion to extracellular matrix components |
| P-selectin | Mediates interactions with other cells, including endothelial cells |
| CD36 | Binds to phosphatidylserine exposed on damaged cells |
This table summarizes some of the key proteins and receptors found on the surface of thrombocytes and their respective functions in platelet activation and hemostasis.
Thrombocyte Activation and Aggregation
Platelets, also known as thrombocytes, are crucial components of the blood’s clotting system. Their activation and subsequent aggregation are essential steps in preventing excessive blood loss following vascular injury. This process is tightly regulated, ensuring that clotting occurs only at the site of damage and not throughout the circulatory system. This process involves a cascade of events, where platelets transform from inactive circulating cells to active participants in the clotting process.The activation of thrombocytes is a complex process involving multiple signaling pathways and interactions between various molecules.
Understanding these mechanisms is vital for comprehending the pathophysiology of thrombosis and for developing therapeutic strategies to prevent or treat thrombotic disorders.
Stimuli Triggering Thrombocyte Activation
Thrombocyte activation is initiated by various stimuli, primarily arising from the damaged endothelium of blood vessels. These stimuli include exposed subendothelial collagen, von Willebrand factor (vWF), and thrombin. The presence of these molecules signals the presence of vascular injury. Furthermore, mechanical stress, such as turbulence in blood flow, can also contribute to platelet activation.
Thrombocytes, also known as platelets, are crucial for blood clotting. They’re tiny cell fragments that help stop bleeding when a blood vessel is damaged. Sometimes, though, you might experience cramps but no period, which can be a sign of various underlying issues. This could include hormonal imbalances or even something more serious. For more information on the causes of cramps but no period, check out this helpful resource: cramps but no period.
Regardless of the cause, understanding thrombocytes is key to overall health and well-being.
Signaling Pathways in Thrombocyte Activation
Platelet activation is mediated by complex intracellular signaling cascades. These pathways involve the activation of intracellular enzymes, such as phospholipase C, which leads to the generation of intracellular messengers like diacylglycerol (DAG) and inositol triphosphate (IP3). These messengers trigger a series of reactions that ultimately lead to platelet shape change, granule release, and aggregation. Key receptors on the platelet surface, including the glycoprotein (GP) Ib/IX/V complex and GP VI, are activated by the presence of these stimuli.
Platelet Aggregation Mechanisms
Platelet aggregation is the process by which platelets adhere to each other and form a plug at the site of vascular injury. This process is crucial for hemostasis. ADP, a crucial mediator of platelet aggregation, is released from activated platelets, binding to ADP receptors on the surface of nearby platelets, stimulating their activation. Thromboxane A2, a potent vasoconstrictor and platelet aggregator, is synthesized and released by activated platelets, further amplifying the aggregation process.
Fibrinogen, a key component of the blood clotting cascade, bridges the activated platelets together, forming a stable aggregate.
Diagram of Platelet Activation and Aggregation
[Imagine a diagram here illustrating the following steps:]
- Vascular injury exposes subendothelial collagen.
- Platelets adhere to exposed collagen via vWF.
- Platelets become activated, changing shape and releasing ADP and thromboxane A2.
- ADP and thromboxane A2 activate more platelets, leading to aggregation.
- Fibrinogen bridges activated platelets, forming a stable aggregate.
Types of Platelet Activation Pathways and Outcomes
| Pathway | Stimuli | Outcomes |
|---|---|---|
| Collagen pathway | Exposed subendothelial collagen | Platelet activation, aggregation, and granule release. |
| Thrombin pathway | Thrombin | Enhanced platelet activation and aggregation, crucial for fibrin formation. |
| ADP pathway | Released ADP | Amplification of platelet activation and aggregation. |
Thrombocyte Disorders
Platelets, or thrombocytes, play a crucial role in blood clotting. Disruptions in their function or number can lead to serious health consequences. Understanding these disorders is vital for accurate diagnosis and effective treatment. This section delves into various conditions related to thrombocyte dysfunction, highlighting their causes, clinical manifestations, and diagnostic approaches.Thrombocyte disorders encompass a spectrum of conditions, ranging from benign variations to life-threatening complications.
These disorders can be broadly categorized as thrombocytopenia (low platelet count) and thrombocytosis (high platelet count). Both conditions can significantly impact the body’s ability to maintain hemostasis and can lead to bleeding or clotting issues, respectively.
Thrombocytopenia
Thrombocytopenia is characterized by a reduced number of circulating platelets. This deficiency impairs the body’s capacity to form blood clots, increasing the risk of bleeding. Various factors contribute to this condition.
Causes of Thrombocytopenia
Several factors can cause a decrease in platelet count. These include:
- Decreased platelet production: Conditions like aplastic anemia, bone marrow failure, and certain medications can inhibit the bone marrow’s ability to produce platelets.
- Increased platelet destruction: Autoimmune disorders, such as immune thrombocytopenic purpura (ITP), lead to the body’s immune system mistakenly attacking and destroying platelets.
- Platelet sequestration: Conditions like hypersplenism, where the spleen sequesters (stores) an excessive number of platelets, can lead to thrombocytopenia.
- Medication-induced thrombocytopenia: Certain medications, like heparin and some chemotherapy drugs, can trigger platelet destruction.
- Infections: Some viral and bacterial infections can also cause thrombocytopenia.
Clinical Manifestations of Thrombocytopenia
The clinical presentation of thrombocytopenia often depends on the severity of the platelet deficiency. Mild cases might be asymptomatic, while severe cases can manifest with:
- Petechiae: Tiny, purplish red spots under the skin, often appearing on the lower extremities.
- Purpura: Larger areas of purplish discoloration on the skin, indicative of bleeding beneath the skin.
- Ecchymosis: Bruising, which appears as a larger area of discoloration.
- Epistaxis: Nosebleeds.
- Bleeding gums: Bleeding from the gums, often with brushing or eating.
- Gastrointestinal bleeding: Bleeding from the digestive tract.
- Intracranial bleeding: Potentially life-threatening bleeding within the skull.
Diagnostic Methods for Thrombocytopenia
Diagnosing thrombocytopenia involves a combination of tests. These include:
- Complete blood count (CBC): A standard blood test that measures various blood components, including platelets.
- Peripheral blood smear: Examination of a blood sample under a microscope to assess platelet morphology and count.
- Bone marrow biopsy: A procedure to examine the bone marrow to evaluate platelet production.
- Coagulation studies: Tests to rule out other bleeding disorders.
- Immune studies: In cases of suspected autoimmune thrombocytopenia, these tests can help identify specific antibodies targeting platelets.
Thrombocytosis
Thrombocytosis is characterized by an elevated platelet count. This condition increases the risk of blood clots, potentially leading to serious complications like stroke or heart attack.
Causes of Thrombocytosis
Several factors can contribute to elevated platelet counts, including:
- Reactive thrombocytosis: An increase in platelets as a response to inflammation, infection, or other underlying medical conditions.
- Essential thrombocythemia: A chronic myeloproliferative disorder characterized by an overproduction of platelets, often requiring ongoing management.
Clinical Manifestations of Thrombocytosis
The clinical manifestations of thrombocytosis can vary depending on the underlying cause and the severity of the elevated platelet count. These can include:
- Thrombosis: Formation of blood clots in blood vessels, potentially leading to stroke, heart attack, or deep vein thrombosis.
- Bleeding: In some cases, thrombocytosis can be associated with bleeding, though this is less common than thrombosis.
Diagnostic Methods for Thrombocytosis
Diagnosing thrombocytosis requires a comprehensive evaluation. This typically involves:
- Complete blood count (CBC): A crucial initial step to measure platelet counts.
- Peripheral blood smear: Examination of blood cells under a microscope for detailed evaluation.
- Bone marrow biopsy: A crucial test to differentiate reactive from essential thrombocytosis.
- Genetic testing: In some cases, genetic testing may be necessary.
Summary Table
| Condition | Causes | Treatment |
|---|---|---|
| Thrombocytopenia (Low Platelets) | Decreased production, increased destruction, sequestration, medication-induced, infections | Treating underlying cause, supportive care, medications like corticosteroids, intravenous immunoglobulin |
| Thrombocytosis (High Platelets) | Reactive (e.g., inflammation, infection), essential thrombocythemia | Treating underlying cause, medications to reduce platelet production, and/or increase platelet destruction |
Clinical Significance of Thrombocytes
Thrombocytes, or platelets, are crucial components of the human body’s intricate system for maintaining hemostasis, the process of preventing and controlling bleeding. Their multifaceted roles extend far beyond simple blood clotting, impacting wound healing, preventing excessive bleeding, and even contributing to the development of potentially life-threatening conditions like thrombosis. Understanding their clinical significance is paramount for accurate diagnosis and effective treatment strategies.Platelets play a vital role in the body’s response to injury.
Their activation triggers a complex cascade of events that lead to the formation of a blood clot, halting blood loss and facilitating tissue repair. This complex interplay between platelets and the coagulation system is essential for maintaining the integrity of the circulatory system.
Importance of Thrombocytes in Wound Healing
Platelets are essential for the initial stages of wound healing. Upon encountering damaged blood vessels, platelets adhere to the exposed collagen and begin aggregating, forming a platelet plug. This temporary plug helps to stop bleeding, allowing for the initiation of the coagulation cascade. Furthermore, platelets release growth factors that stimulate the proliferation of fibroblasts and the production of extracellular matrix, contributing to the formation of granulation tissue and the eventual healing of the wound.
Their role in the formation of new blood vessels (angiogenesis) also facilitates the supply of nutrients and oxygen to the injured site, promoting tissue regeneration.
Role of Thrombocytes in Preventing Excessive Bleeding
Platelets are integral to the process of hemostasis, a crucial mechanism for preventing excessive bleeding. Their ability to adhere to damaged blood vessel walls and aggregate to form a platelet plug is fundamental in this process. This initial plug, along with the subsequent activation of the coagulation cascade, effectively halts bleeding. This process is vital in preventing life-threatening hemorrhage following trauma or surgical procedures.
Connection Between Thrombocytes and Thrombosis
While platelets are essential for preventing bleeding, their inappropriate activation and aggregation can lead to thrombosis, a condition characterized by the formation of blood clots within blood vessels. In certain situations, factors such as high blood pressure, hyperlipidemia, or inflammation can trigger excessive platelet activation, leading to the formation of thrombi that can obstruct blood flow. This can have serious consequences, potentially leading to heart attacks, strokes, or deep vein thrombosis.
Understanding the delicate balance between platelet activation and inhibition is crucial for preventing and treating thrombosis.
Clinical Scenarios Requiring Thrombocyte Count Assessment
Platelet counts are essential diagnostic and treatment indicators in numerous clinical scenarios. Anomalies in platelet counts can provide valuable insights into underlying conditions.
| Clinical Scenario | Significance of Thrombocyte Count |
|---|---|
| Thrombocytopenia | Low platelet count can indicate various conditions like bone marrow disorders, autoimmune diseases, or infections. |
| Thrombocytosis | Elevated platelet count may suggest conditions like myeloproliferative disorders, inflammatory conditions, or iron deficiency. |
| Bleeding Disorders | Low platelet counts are frequently associated with bleeding disorders, affecting the body’s ability to form blood clots. |
| Post-Surgery/Trauma | Monitoring platelet counts is critical after surgery or trauma to assess the risk of bleeding complications. |
| Cancer Treatment | Platelet counts can be affected by chemotherapy and radiation therapy, impacting treatment protocols. |
Role of Thrombocytes in the Coagulation Cascade
Platelets are critical participants in the coagulation cascade, the complex series of biochemical reactions leading to the formation of a blood clot. Their activation initiates this cascade, amplifying the process through the release of various factors that promote clot formation. The coagulation cascade, initiated by platelets, culminates in the formation of a fibrin mesh, which stabilizes the platelet plug and effectively stops bleeding.
The precise control of this cascade is essential to maintain hemostasis and prevent uncontrolled clotting.
Thrombocytes in Disease States
Platelets, or thrombocytes, are crucial for hemostasis, the process that stops bleeding. However, imbalances in platelet numbers or function can lead to a range of serious diseases. Understanding their role in various pathological conditions is vital for effective diagnosis and treatment.
Cardiovascular Diseases
Platelets play a pivotal role in the development and progression of cardiovascular diseases, particularly atherosclerosis and thrombosis. In atherosclerosis, the build-up of plaque in the arteries can trigger platelet activation. Activated platelets adhere to the damaged endothelium, forming a platelet plug that contributes to the growth of the plaque and narrowing of the arteries. This process can lead to reduced blood flow and increase the risk of myocardial infarction (heart attack) or stroke.
Thrombosis, the formation of blood clots, is another crucial aspect of cardiovascular disease. Platelets are essential components of thrombi, or blood clots, which can obstruct blood vessels and cause severe complications. Abnormal platelet function, such as increased aggregation or decreased clearance, can increase the risk of thrombosis.
So, thrombocytes, also known as platelets, are crucial for blood clotting. They’re tiny cell fragments that help stop bleeding when you get a cut. Thinking about the amazing ways our bodies work, I was wondering if there was a connection between healthy blood and the benefits of celery juice. Apparently, some people believe that celery juice can help boost overall health, potentially even supporting healthy blood function.
To learn more about what celery juice is good for, check out this informative article what is celery juice good for. Regardless of the claims, thrombocytes remain an essential part of the blood clotting process.
Inflammatory Conditions
Platelets are increasingly recognized as active participants in inflammatory responses. They can release various mediators that modulate inflammation. In some inflammatory conditions, such as rheumatoid arthritis, an overabundance of activated platelets can contribute to tissue damage and inflammation. The release of inflammatory mediators from platelets amplifies the inflammatory cascade, potentially exacerbating the disease process. In other inflammatory conditions, platelets may play a protective role by limiting the extent of inflammation.
Cancers and Malignancies
Thrombocytosis, an abnormally high platelet count, is frequently associated with certain cancers and malignancies. Cancer cells can stimulate platelet production or alter platelet function. Platelet activation in the context of cancer can contribute to tumor growth and metastasis. Moreover, platelets can provide a supportive microenvironment for cancer cell growth and dissemination.
Thrombocyte Abnormalities in Various Diseases
| Disease | Thrombocyte Abnormality | Mechanism |
|---|---|---|
| Atherosclerosis | Increased platelet activation and aggregation | Platelets adhere to damaged endothelium, contributing to plaque formation and narrowing of arteries. |
| Thrombosis | Increased platelet aggregation and clot formation | Platelets form a major component of thrombi, which can obstruct blood vessels. |
| Rheumatoid Arthritis | Increased platelet activation and release of inflammatory mediators | Platelets contribute to tissue damage and inflammation in the joints. |
| Cancer | Thrombocytosis or altered platelet function | Cancer cells can stimulate platelet production or alter platelet function, potentially contributing to tumor growth and metastasis. |
| Immune Thrombocytopenic Purpura (ITP) | Decreased platelet count | Autoimmune destruction of platelets by the immune system. |
| Thrombotic Thrombocytopenic Purpura (TTP) | Decreased platelet count, microangiopathic hemolytic anemia | Defective ADAMTS13 enzyme leading to the formation of large von Willebrand factor multimers that cause microthrombi and consumption of platelets. |
Thrombocyte Research and Future Directions
Platelets, or thrombocytes, play a crucial role in hemostasis, the process that stops bleeding. Understanding their complex functions, from activation and aggregation to the regulation of their lifespan, is essential for developing effective treatments for bleeding disorders and thrombotic diseases. Recent research has significantly advanced our knowledge of thrombocytes, opening doors to novel therapeutic approaches.Current research efforts are focusing on elucidating the intricate mechanisms governing platelet function, from their formation and maturation to their activation and clearance.
This deeper understanding will lead to the development of more targeted therapies and personalized medicine approaches for various conditions involving platelets.
Current Research Areas
Research into thrombocytes encompasses a broad spectrum of areas, each contributing to a more comprehensive understanding of these vital cellular components. Studies are exploring the intricate signaling pathways involved in platelet activation, aiming to identify key molecular targets for therapeutic intervention. Investigating the role of platelets in inflammation and immune responses is also gaining momentum, revealing their involvement in a wider range of physiological processes than previously thought.
Furthermore, researchers are actively investigating the genetic basis of platelet disorders, aiming to develop diagnostic tools and personalized treatment strategies.
Challenges in Thrombocyte Research
Despite significant advancements, several challenges remain in thrombocyte research. One major hurdle is the complexity of platelet activation and aggregation, involving a cascade of intricate interactions between different proteins and signaling molecules. This complexity makes it challenging to identify specific targets for therapeutic interventions without affecting other crucial physiological processes. Another challenge lies in developing reliable and reproducible in vitro models that accurately reflect the in vivo behavior of platelets.
Furthermore, ethical considerations surrounding the use of human platelets in research and the development of novel therapies need careful consideration.
Opportunities in Thrombocyte Research
Despite the challenges, the field of thrombocyte research offers exciting opportunities. The development of sophisticated imaging techniques allows for real-time visualization of platelet function in vivo, providing valuable insights into their behavior in different physiological contexts. The use of advanced bioengineering techniques, such as 3D cell culture models, is helping to create more accurate representations of platelet function in various disease states.
The increasing availability of genomic and proteomic data is paving the way for personalized medicine approaches tailored to individual patient needs.
Novel Therapies Targeting Thrombocytes
The potential for novel therapies targeting thrombocytes is immense. Anti-platelet agents are already widely used in clinical practice for the prevention of cardiovascular events. However, the development of more specific and targeted therapies, potentially including therapies that modulate the lifespan or activation state of platelets, is a key area of focus. Researchers are exploring the use of gene therapy and other advanced therapeutic approaches to correct platelet function defects in inherited bleeding disorders.
The design of drugs that specifically inhibit or enhance platelet function in a targeted manner holds significant promise for the future.
Advancements in Understanding Thrombocyte Function
Significant progress has been made in understanding the intricate mechanisms underlying platelet function. For instance, advancements in proteomics have identified numerous proteins involved in platelet activation and signaling. Studies on the role of platelet microparticles and their impact on vascular function are further expanding our understanding of the broader role of platelets in physiological processes. This enhanced knowledge is instrumental in developing more targeted therapies and improving diagnostic capabilities.
Future Research Directions
Future research in thrombocyte biology should focus on developing novel strategies for personalized medicine. This includes tailoring therapies based on individual patient characteristics, including genetic predispositions and disease phenotypes. Another critical area is the development of new diagnostic tools for the early detection and monitoring of platelet disorders. Furthermore, research should explore the potential of platelet-based therapies for treating a wider range of diseases, such as cancers and inflammatory conditions.
Ultimately, a deeper understanding of platelet biology will pave the way for improved treatments and preventive strategies for a variety of conditions.
Wrap-Up
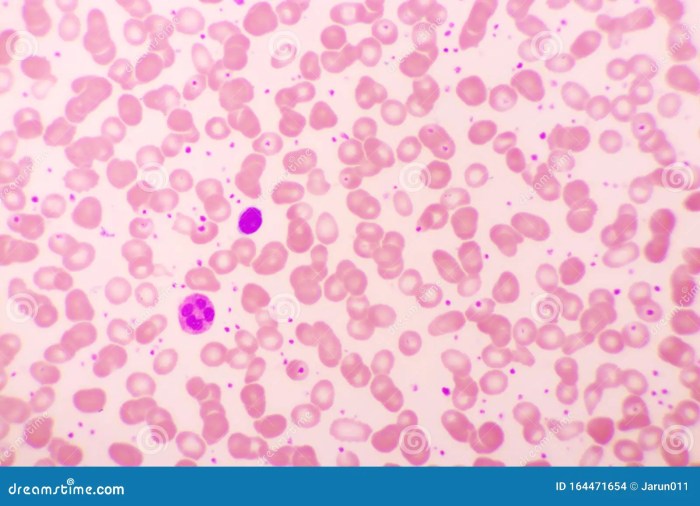
In conclusion, thrombocytes, or platelets, are essential components of the human body’s intricate system for maintaining hemostasis and preventing excessive bleeding. Their formation, structure, function, and involvement in various diseases highlight their critical role in wound healing and overall health. Further research into these remarkable cells promises to unlock even more insights into their complex mechanisms and potential therapeutic applications.