Psoriatic disease drug pipeline offers a fascinating look at the innovative treatments emerging for this chronic condition. From understanding the various types of psoriasis and their unmet needs, to examining current therapies and their limitations, we delve into the promising pipeline of new drugs. This exploration covers the stages of development, potential benefits, and associated risks, all the way to future directions and the role of personalized medicine.
This in-depth analysis provides a comprehensive overview of the current state of the psoriatic disease drug pipeline, including a detailed look at emerging therapies, clinical trial methodologies, and the challenges and opportunities in development. We’ll analyze the potential of new approaches like biologics, small molecules, and gene therapies, while also considering the crucial aspects of clinical trial design and regulatory considerations.
Overview of Psoriatic Disease
Psoriatic disease is a chronic autoimmune condition characterized by inflammation and skin changes. It’s more than just a skin condition; it affects multiple organ systems and can lead to significant physical and psychological distress. Understanding the diverse presentations and unmet needs is crucial for improving patient outcomes.Psoriatic disease encompasses a spectrum of conditions, each with its own clinical characteristics.
While skin manifestations are often prominent, the disease can also involve joints, nails, and internal organs. Current treatments, while effective for some, frequently fall short in achieving complete remission or preventing long-term complications. This leaves a substantial unmet need for more targeted and effective therapies.
Types of Psoriatic Disease
Psoriatic disease manifests in various forms, with overlapping symptoms and potentially different underlying mechanisms. Recognizing these distinctions is vital for personalized treatment strategies.
- Plaque psoriasis: This is the most common type, characterized by raised, red, scaly patches on the skin. These lesions are often itchy and painful, and can vary in size and location.
- Inverse psoriasis: This form typically appears in skin folds, such as the groin, armpits, and under the breasts. It presents as smooth, red, and shiny patches, often mistaken for other conditions.
- Guttate psoriasis: Small, drop-like lesions appear on the skin, often following an infection, such as strep throat. These lesions can be widespread or localized.
- Erythrodermic psoriasis: This is a severe form characterized by widespread redness and scaling of the skin, affecting over 90% of the body surface. It requires immediate medical attention due to its significant systemic impact.
- Psoriatic arthritis: This type involves inflammation of the joints, leading to pain, stiffness, and swelling. It can affect any joint, but commonly affects the fingers, toes, spine, and large joints. The joint involvement can range from mild to severe, and may occur before, simultaneously with, or after skin manifestations.
Clinical Presentations
The clinical presentations of psoriatic disease can vary significantly among individuals, making diagnosis and treatment challenging. Factors such as genetic predisposition, environmental triggers, and the specific type of psoriatic disease all play a role.
- Skin manifestations: Varying degrees of redness, scaling, and inflammation on the skin surface. Nail involvement can include pitting, discoloration, and separation from the nail bed. The specific appearance and location of skin lesions can differ between individuals and disease types.
- Joint involvement: Psoriatic arthritis can cause pain, stiffness, and swelling in the joints. It can range from mild to severe, leading to significant functional limitations.
- Systemic manifestations: Some patients with psoriatic disease experience systemic symptoms, including fatigue, fever, and weight loss. These symptoms may reflect the systemic inflammation associated with the disease.
Unmet Medical Needs
Current treatments for psoriatic disease often fail to achieve complete remission or prevent long-term complications. This highlights the need for improved therapeutic approaches.
- Lack of effective treatments for severe and recalcitrant forms: Effective treatments for individuals with severe or recalcitrant disease are limited, leading to significant patient burden and potential complications.
- Treatment side effects: Some existing treatments have notable side effects, including immunosuppression, which can increase the risk of infections. Development of therapies with a reduced risk of adverse events is crucial.
- Personalized treatment approaches: A deeper understanding of the underlying mechanisms of psoriatic disease is needed to tailor treatment strategies to individual patient needs.
Global Prevalence and Impact
Psoriatic disease affects millions worldwide, posing a significant public health concern. The chronic nature of the disease and its impact on quality of life create a substantial burden on healthcare systems.
Table of Psoriatic Disease Types
| Type of Psoriatic Disease | Symptoms | Typical Treatments |
|---|---|---|
| Plaque Psoriasis | Raised, red, scaly patches on skin; itchy and painful | Topical corticosteroids, vitamin D analogs, salicylic acid, phototherapy, systemic medications (methotrexate, biologics) |
| Inverse Psoriasis | Smooth, red, shiny patches in skin folds | Topical corticosteroids, topical calcineurin inhibitors, phototherapy, systemic medications |
| Guttate Psoriasis | Small, drop-like lesions on skin, often following infection | Topical corticosteroids, phototherapy, systemic medications (if severe) |
| Erythrodermic Psoriasis | Widespread redness and scaling of skin, affecting over 90% of body surface | Hospitalization, systemic medications, intensive phototherapy |
| Psoriatic Arthritis | Inflammation of joints, causing pain, stiffness, and swelling | NSAIDs, DMARDs (methotrexate, sulfasalazine), biologics, physical therapy |
Current Therapies for Psoriatic Disease
Psoriatic disease, a chronic inflammatory condition, presents a complex challenge for treatment. Effective therapies aim to reduce inflammation, control skin lesions, and alleviate associated symptoms. Understanding the diverse range of current treatment options, their limitations, and efficacy profiles is crucial for patients and healthcare providers.
Existing Therapies Categorized by Mechanism of Action
Current treatments for psoriatic disease target various inflammatory pathways and mechanisms within the body. These therapies are often categorized based on their primary mode of action. Different classes of medications address the underlying causes of the disease in various ways, offering patients a range of treatment options.
- Topical Treatments: These therapies are often the first line of defense for mild to moderate psoriasis. They directly apply medication to the affected skin, minimizing systemic exposure and potentially reducing side effects. Topical treatments can include corticosteroids, vitamin D analogs, and retinoids, each with distinct mechanisms of action. Corticosteroids reduce inflammation, while vitamin D analogs modulate immune responses, and retinoids promote skin cell turnover.
- Systemic Treatments: These therapies are reserved for moderate to severe cases where topical treatments are insufficient. Systemic medications, taken orally or intravenously, can achieve broader effects throughout the body, potentially controlling inflammation more effectively. Examples include methotrexate, cyclosporine, and TNF inhibitors. Methotrexate is an immunosuppressant, cyclosporine suppresses the immune system, and TNF inhibitors target tumor necrosis factor-alpha, a key inflammatory cytokine.
The psoriatic disease drug pipeline is buzzing with promising new treatments, but understanding potential side effects is crucial. For instance, some medications can lead to reactive hypoglycemia, a condition where blood sugar drops suddenly after eating. Learning more about this, including symptoms and management strategies, can be extremely helpful for patients considering these new therapies. For more in-depth information on reactive hypoglycemia, check out this helpful resource: what to know about reactive hypoglycemia.
Ultimately, staying informed about both the exciting advancements and potential complications in the psoriatic disease drug pipeline is key for making the best decisions about treatment.
- Phototherapy: Phototherapy utilizes ultraviolet (UV) light to target and reduce skin inflammation. Different types of UV light, such as UVB and UVA, are used in various modalities, including narrowband UVB, PUVA (psoralen plus UVA), and excimer laser therapy. Phototherapy is particularly effective in managing certain forms of psoriasis, and the specific type of light and dosage is tailored to the individual’s needs.
- Biologics: Biologics are a newer class of systemic treatments that target specific proteins or pathways involved in the inflammatory response. These medications, often administered by injection, can significantly reduce inflammation and improve skin clearance. Examples include interleukin-12/23 inhibitors, and anti-TNF agents. Biologics have proven to be highly effective for many patients, but they can carry potential risks.
Limitations and Drawbacks of Current Treatments
While existing therapies offer varying degrees of success, they also have limitations and drawbacks. Side effects, efficacy variability, and cost considerations can influence treatment decisions. Not all treatments are suitable for every patient, and factors such as overall health, other medical conditions, and patient preferences need to be considered.
- Side Effects: Systemic treatments, particularly immunosuppressants, can have significant side effects, including increased risk of infections, liver damage, and other organ toxicity. Topical treatments can also cause skin irritation or allergic reactions. The specific side effects and their severity vary significantly between individuals and the type of medication.
- Efficacy Variability: The effectiveness of various treatments can vary considerably among patients. Some individuals may respond well to one therapy but not another. The severity and type of psoriasis can also influence treatment response. Individualized treatment plans are essential to maximize efficacy.
- Cost Considerations: Some biologic therapies can be very expensive, making them inaccessible to some patients. The long-term costs of treatment need to be considered alongside the potential benefits and risks.
Efficacy and Safety Profiles of Existing Treatments
The efficacy and safety profiles of psoriatic disease treatments are crucial factors in choosing the most appropriate approach. Evidence-based studies provide data on the effectiveness of various therapies in reducing disease activity and improving quality of life. Clinical trials and real-world data contribute to our understanding of treatment outcomes.
Comparison of Treatment Approaches
Different treatment approaches offer varying degrees of efficacy and side effects. Topical treatments are often less aggressive but may not be sufficient for severe cases. Systemic treatments can be more effective but come with greater potential side effects. Biologics and phototherapies represent a balance between efficacy and potential risks.
Table Contrasting Treatment Options
| Treatment Type | Mechanism of Action | Efficacy | Side Effects | Cost |
|---|---|---|---|---|
| Topical Corticosteroids | Reduce inflammation | Moderate for mild-moderate cases | Skin irritation, thinning, atrophy | Low |
| Systemic Methotrexate | Immunosuppressant | High for moderate-severe cases | Liver damage, nausea, mouth sores | Moderate |
| Biologics (e.g., TNF inhibitors) | Target inflammatory pathways | High for severe cases | Increased risk of infection, allergic reactions | High |
| Phototherapy (UVB) | Reduce inflammation via UV light | Moderate to high | Skin reactions, premature aging | Moderate |
Drug Pipeline Analysis: Psoriatic Disease Drug Pipeline
The psoriatic disease drug pipeline is a dynamic landscape, constantly evolving with new therapies emerging. Understanding the current pipeline is crucial for patients, clinicians, and researchers alike, as it offers glimpses into future treatment options and potential breakthroughs in managing this complex condition. This analysis delves into the specifics of these promising drugs, exploring their stages of development, mechanisms of action, and potential impact on the lives of those affected by psoriasis.
Drugs in the Pipeline
The psoriatic disease drug pipeline encompasses a diverse range of therapies, targeting various aspects of the disease process. These therapies aim to address different facets of psoriasis, including inflammation, cell growth, and immune system regulation. Understanding the variety of approaches is vital for evaluating the potential of these drugs.
- Biologics targeting specific inflammatory pathways: Several biologics are in development, designed to target specific inflammatory pathways implicated in psoriasis pathogenesis. These agents often show high efficacy in clinical trials and aim to reduce inflammation and associated symptoms, potentially with fewer side effects compared to traditional therapies. Examples include monoclonal antibodies that block specific cytokines involved in the inflammatory response.
- Small molecule inhibitors: Small molecule inhibitors represent another promising area of development. These compounds often have different mechanisms of action, aiming to modulate key enzymes or proteins implicated in the disease. Small molecule inhibitors have the potential for oral administration, which can significantly improve patient convenience and adherence. A notable example could be a drug targeting a specific enzyme responsible for cell proliferation.
- Combination therapies: Combination therapies, involving the combination of two or more existing or emerging treatments, show potential for enhanced efficacy and reduced side effects. This approach could be particularly beneficial for patients who do not respond adequately to monotherapy. For instance, a combination of a biologic and a small molecule inhibitor could offer a more comprehensive approach to disease management.
- Topical therapies with novel mechanisms: Topical therapies, designed to target the skin directly, are also in development. These therapies aim to address local inflammation and reduce symptoms while minimizing systemic side effects. One promising example is a topical cream containing a novel compound that targets specific signaling pathways involved in skin inflammation.
Stage of Development
The stage of development for each drug varies, ranging from preclinical studies to late-stage clinical trials. Understanding the progress of each drug is essential for assessing its potential and timeline for market entry.
- Preclinical studies: Preclinical studies involve laboratory experiments and animal models to evaluate the safety and efficacy of a drug candidate before human testing. These studies help to identify potential risks and determine the optimal dosage regimen for future clinical trials. These initial phases are crucial for ensuring that the drug is both safe and potentially effective before human testing begins.
- Clinical trials: Clinical trials assess the drug’s safety and efficacy in human subjects. These trials are typically divided into phases, with each phase building upon the previous one. Phase 1 focuses on safety, Phase 2 on efficacy and dosing, and Phase 3 on confirming efficacy and comparing it to existing treatments.
Mechanism of Action, Psoriatic disease drug pipeline
Understanding the mechanism of action for each drug is critical for evaluating its potential benefits and risks. The specific mechanism of action often dictates the drug’s target within the disease process.
- Biologics: Biologics typically target specific proteins or cytokines involved in the inflammatory response, such as TNF-α or IL-17. This targeting can significantly reduce inflammation and symptoms, often leading to improved skin clearance.
- Small molecule inhibitors: Small molecule inhibitors can target different pathways involved in cell growth, differentiation, or immune response. These drugs can often exert their effects through modulation of specific enzymes or proteins, which can have a more targeted impact on the disease process.
Potential Benefits and Risks
The potential benefits of new therapies are usually weighed against the potential risks. Factors such as efficacy, safety profile, and patient convenience are essential considerations.
Clinical Trial Phases
| Phase | Description | Number of Patients (Example) |
|---|---|---|
| Phase 1 | Safety and tolerability testing | 20-80 |
| Phase 2 | Efficacy and dose finding | 50-300 |
| Phase 3 | Large-scale efficacy and safety comparison | 300-3000 |
Emerging Therapies and Novel Approaches
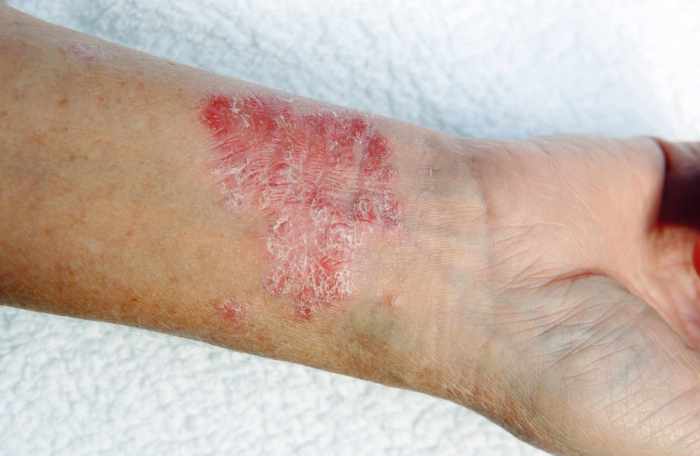
The psoriatic disease landscape is constantly evolving, with innovative therapies pushing the boundaries of treatment. Beyond traditional approaches, emerging strategies are promising to deliver more effective and targeted interventions, improving patient outcomes and quality of life. These therapies leverage advancements in understanding the disease mechanisms and offer hope for a future where psoriatic disease can be better managed and potentially even cured.
Biologics: Refining Targeted Immunotherapy
Biologics have revolutionized the treatment of psoriatic disease by targeting specific components of the immune system involved in the inflammatory response. These therapies, often monoclonal antibodies, precisely modulate immune pathways, leading to a reduction in inflammation and skin lesions. They typically exhibit a faster onset of action and greater efficacy compared to conventional therapies. Examples include TNF-alpha inhibitors (e.g., infliximab, etanercept), interleukin-12/23 inhibitors (e.g., ustekinumab), and anti-interleukin-17 inhibitors (e.g., secukinumab).
Small Molecules: Expanding Treatment Options
Small molecules represent another promising avenue in the development of psoriatic disease therapies. These drugs, typically oral medications, have the potential to offer a more convenient treatment regimen compared to biologics, potentially reducing the need for injections. Small molecules often work by inhibiting specific enzymes or pathways involved in inflammation. Examples include apremilast, which targets phosphodiesterase-4, and tofacitinib, a Janus kinase inhibitor.
The advantage of small molecules lies in their oral administration and potential for broader applicability.
Gene Therapies: A Novel Frontier
Gene therapies represent a cutting-edge approach to psoriatic disease treatment. These therapies aim to modify the underlying genetic defects contributing to the disease process. This innovative approach has the potential to offer long-term disease control or even a cure. One promising example involves the use of gene editing technologies like CRISPR to target specific genes involved in inflammation.
Early-stage clinical trials are exploring the safety and efficacy of these approaches.
The psoriatic disease drug pipeline is buzzing with exciting new developments, promising better treatments for those affected. Finding ways to manage the symptoms of skin conditions like rosacea is crucial, and understanding how to deal with papulopustular rosacea, for example, how to deal with papulopustular rosacea , is key. Ultimately, advancements in this pipeline are driving hope for more effective and targeted therapies for psoriatic diseases.
Table: Comparison of Emerging Therapies
| Therapy Type | Mechanism of Action | Potential Benefits | Safety Profile |
|---|---|---|---|
| Biologics | Target specific immune components, modulating inflammatory pathways | Faster onset, greater efficacy, often fewer side effects than traditional therapies | Potential for rare but serious side effects, including infections and allergic reactions. Careful monitoring required. |
| Small Molecules | Inhibit enzymes or pathways involved in inflammation, often orally administered | Convenience of oral administration, potentially broader applicability | Potential for side effects, including gastrointestinal issues, liver problems, or increased risk of infections |
| Gene Therapies | Modify underlying genetic defects contributing to the disease process | Potential for long-term disease control or cure | Still under development, safety concerns remain. Potential for off-target effects or immune reactions. |
Clinical Trial Design and Methodology
Unveiling the intricate process of evaluating new psoriatic disease treatments requires meticulous planning and execution. Clinical trials provide a structured approach to assess the safety and efficacy of novel drugs, ensuring their suitability for widespread use. This meticulous process safeguards patient well-being and guarantees the credibility of the results.
Common Clinical Trial Designs
Clinical trials employ various designs to evaluate the efficacy and safety of treatments. Randomized controlled trials (RCTs) are a cornerstone of this process, assigning patients randomly to either a treatment group or a control group. This randomization minimizes bias and allows for a fair comparison of treatment outcomes. Other designs, such as observational studies, can offer valuable insights into the real-world application of therapies.
This allows researchers to see how the drug behaves in a less controlled environment.
Patient Selection Criteria
Rigorous criteria are applied to select patients for clinical trials. These criteria ensure that the study participants are representative of the population likely to benefit from the treatment. Factors like the type and severity of psoriasis, the presence of comorbidities, and previous treatment responses influence the selection process. Inclusion and exclusion criteria are meticulously defined to ensure the study’s results are relevant and applicable to a wider population.
Endpoints for Measuring Treatment Efficacy
Several endpoints are employed to assess treatment efficacy in clinical trials. These endpoints encompass various aspects of the disease, including the extent of skin involvement, the degree of inflammation, and the impact on patient quality of life. Objective measures, such as skin lesion area and severity, are often complemented by patient-reported outcomes, capturing the subjective experience of the disease and treatment.
Ethical Considerations and Regulatory Requirements
Clinical trials are subject to stringent ethical guidelines and regulatory requirements. These regulations prioritize patient safety and well-being. Informed consent is crucial, ensuring that patients understand the potential risks and benefits of participating in the trial. Independent ethics committees (IECs) review the trial protocol to ensure its ethical soundness. Adherence to Good Clinical Practice (GCP) standards is mandatory to maintain the integrity and quality of the data collected.
Table: Phases of Clinical Trials
| Phase | Primary Endpoints | Secondary Endpoints |
|---|---|---|
| Phase I | Safety and tolerability of the drug in a small group of healthy volunteers or patients with the disease. | Preliminary evidence of efficacy in a limited patient population. Potential side effects are observed and dosage is refined. |
| Phase II | Determining the optimal dosage and assessing the drug’s effectiveness in a larger group of patients. | Further exploration of safety, identification of specific patient subgroups who may benefit most, and comparison of different treatment approaches. |
| Phase III | Comparing the new drug to existing standard treatments in a large, diverse patient population. | Evaluating long-term safety, efficacy, and side effects, identifying any differences in response based on patient characteristics, and comparing the drug to existing treatments for a definitive result. |
| Phase IV | Monitoring the long-term effects of the drug in a broader patient population after it has been approved. | Identifying rare side effects, confirming efficacy in various populations, and exploring different ways to use the drug. Real-world effectiveness and optimal patient management strategies are also explored. |
Challenges and Opportunities in Psoriatic Disease Drug Development
Navigating the complex landscape of psoriatic disease requires innovative therapies to address the diverse clinical presentations and unmet needs of patients. While significant progress has been made in the development of biologics and small molecules, hurdles remain in achieving optimal efficacy, minimizing side effects, and expanding treatment options for various subtypes of the disease. This discussion delves into the key challenges and opportunities within the psoriatic disease drug pipeline.
Key Challenges in Developing New Therapies
Developing effective and safe therapies for psoriatic disease is challenging due to the complex pathophysiology of the disease. Psoriasis involves multiple immune pathways and inflammatory processes, making it difficult to target specific mechanisms without significant off-target effects. Furthermore, the heterogeneity of disease presentation, including plaque psoriasis, guttate psoriasis, and inverse psoriasis, necessitates tailored treatments for each subtype. Developing therapies that effectively address all these variations poses a significant hurdle.
Regulatory Hurdles in Bringing New Drugs to Market
The regulatory pathway for new drugs is rigorous and demanding. Demonstrating both efficacy and safety across diverse patient populations is crucial for approval. Extensive clinical trials are necessary to gather robust data on the drug’s performance, side effects, and long-term impact. Meeting stringent regulatory requirements, often involving multiple phases of trials and extensive documentation, significantly delays and increases the cost of bringing a new drug to market.
Meeting these criteria is critical to ensure the safety and efficacy of the drug for patients.
Financial Considerations Impacting Drug Development
The cost of developing a new drug is substantial, encompassing research, clinical trials, regulatory submissions, and manufacturing. The high financial burden often deters pharmaceutical companies from pursuing research into less commercially promising areas. The financial risk associated with drug development also impacts the investment in innovative approaches, as companies often prioritize drugs with higher potential returns. This may lead to a lack of exploration into certain areas that could potentially yield significant breakthroughs in the long term.
Potential Opportunities for Innovation in the Psoriatic Disease Drug Pipeline
Several innovative approaches hold promise in addressing the challenges of psoriatic disease treatment. Targeted therapies that selectively modulate specific immune pathways are being explored, aiming to achieve greater efficacy and reduce side effects. Combination therapies, where multiple drugs are used in conjunction, offer the potential to enhance treatment outcomes by targeting multiple aspects of the disease. Precision medicine approaches, leveraging patient-specific genetic information to tailor treatments, represent another promising area for innovation.
Recent advancements in the psoriatic disease drug pipeline are exciting, promising new treatments for those living with this condition. However, it’s important to consider the broader context of chronic pain management, particularly when exploring conditions like the phantom pain discussed in spotlight on phantom pain. This nuanced understanding of pain management is crucial as we continue to develop innovative treatments within the psoriatic disease drug pipeline.
These approaches could potentially lead to more effective and personalized therapies.
Table: Challenges and Opportunities in Psoriatic Disease Drug Development
| Challenges | Opportunities |
|---|---|
| Complex pathophysiology and heterogeneity of disease presentation | Targeted therapies, combination therapies, precision medicine |
| Rigorous regulatory requirements and extensive clinical trials | Streamlined regulatory pathways, innovative trial designs |
| High financial burden and risk associated with drug development | Public-private partnerships, innovative financing models, focus on high-impact targets |
| Limited understanding of disease mechanisms | Advanced research technologies, interdisciplinary collaborations |
Future Directions and Predictions
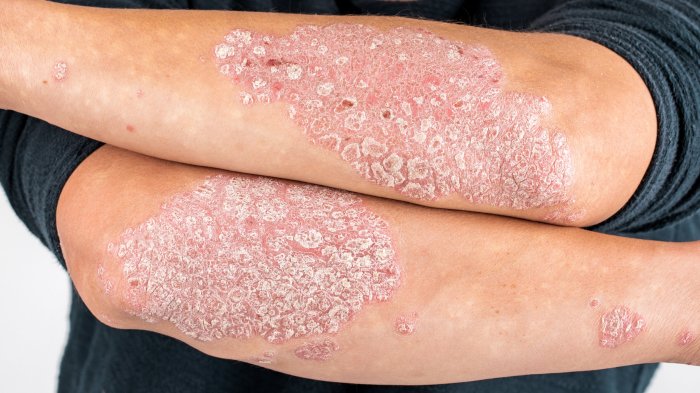
The psoriatic disease drug pipeline is brimming with exciting possibilities, promising significant advancements in patient care. Recent breakthroughs in understanding the complex interplay of genetics, immunology, and environmental factors contributing to the disease are driving the development of innovative therapies. These developments are paving the way for more effective and targeted treatments, potentially revolutionizing how psoriatic disease is managed.The future of psoriatic disease drug development hinges on several key areas, including personalized medicine, combination therapies, and the exploration of novel targets.
This evolving landscape will likely see a shift towards more tailored approaches, emphasizing the individual needs of each patient. Furthermore, the convergence of multiple therapeutic strategies will potentially lead to superior outcomes and reduced side effects.
Personalized Medicine Approaches
Personalized medicine strategies are poised to become increasingly important in the treatment of psoriatic disease. This involves tailoring treatment plans based on a patient’s unique genetic profile, disease characteristics, and individual response to various therapies. By understanding the specific genetic predispositions and immune responses of individual patients, clinicians can select the most effective therapies and minimize adverse reactions.
For example, genetic testing could identify patients who are more likely to respond positively to specific biologics, allowing for more targeted and efficient treatment strategies.
Combination Therapies
The use of combination therapies is another promising avenue for enhancing treatment efficacy and reducing the potential for resistance development. Combining different drugs with complementary mechanisms of action could lead to synergistic effects, effectively suppressing the inflammatory response associated with psoriatic disease. This approach may also help mitigate the side effects often associated with single-agent therapies.
Potential Future Treatment Combinations
| Treatment Combination | Projected Outcomes |
|---|---|
| Biologic plus topical corticosteroid | Potentially enhanced efficacy with reduced systemic side effects. |
| JAK inhibitor plus phototherapy | Improved efficacy, potentially reducing the need for high-dose phototherapy. |
| Anti-TNFα agent plus IL-17 inhibitor | Synergistic reduction in inflammation, possibly leading to a more complete resolution of skin lesions. |
| IL-23 inhibitor plus topical retinoid | Improved efficacy in patients with moderate-to-severe disease, with a potential for improved skin quality and reduced inflammation. |
| Small molecule inhibitor plus vitamin D analogue | Potential for reduced systemic side effects and improved efficacy, particularly in patients with mild to moderate disease. |
The table above illustrates potential combinations of treatments and their projected outcomes. However, these are preliminary projections and need to be confirmed through rigorous clinical trials.
Novel Approaches
Beyond traditional biologics and small molecules, novel therapeutic approaches are emerging. These approaches include targeting novel pathways involved in psoriatic disease pathogenesis, such as specific immune cell interactions, cytokine networks, and cellular signaling cascades. These advancements will likely lead to more effective and targeted treatments.
Closure
In conclusion, the psoriatic disease drug pipeline presents a dynamic landscape of hope for patients facing this chronic condition. The development of new therapies, while facing challenges in clinical trials and regulatory hurdles, offers a promising future for improved treatment outcomes. The potential of personalized medicine and innovative approaches suggests a path toward more effective and targeted therapies, with significant implications for patient quality of life.
Continued research and development in this area are critical to realizing the full potential of these new treatments.